Participants
Mobile Phone
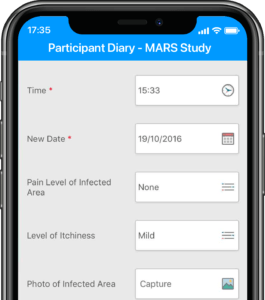
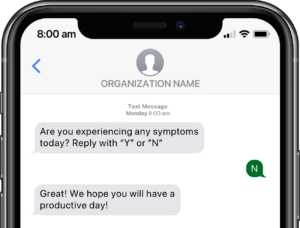
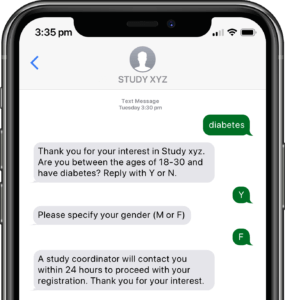
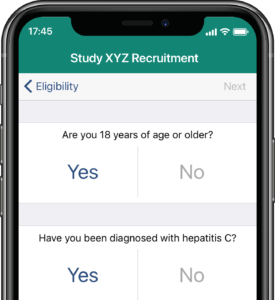
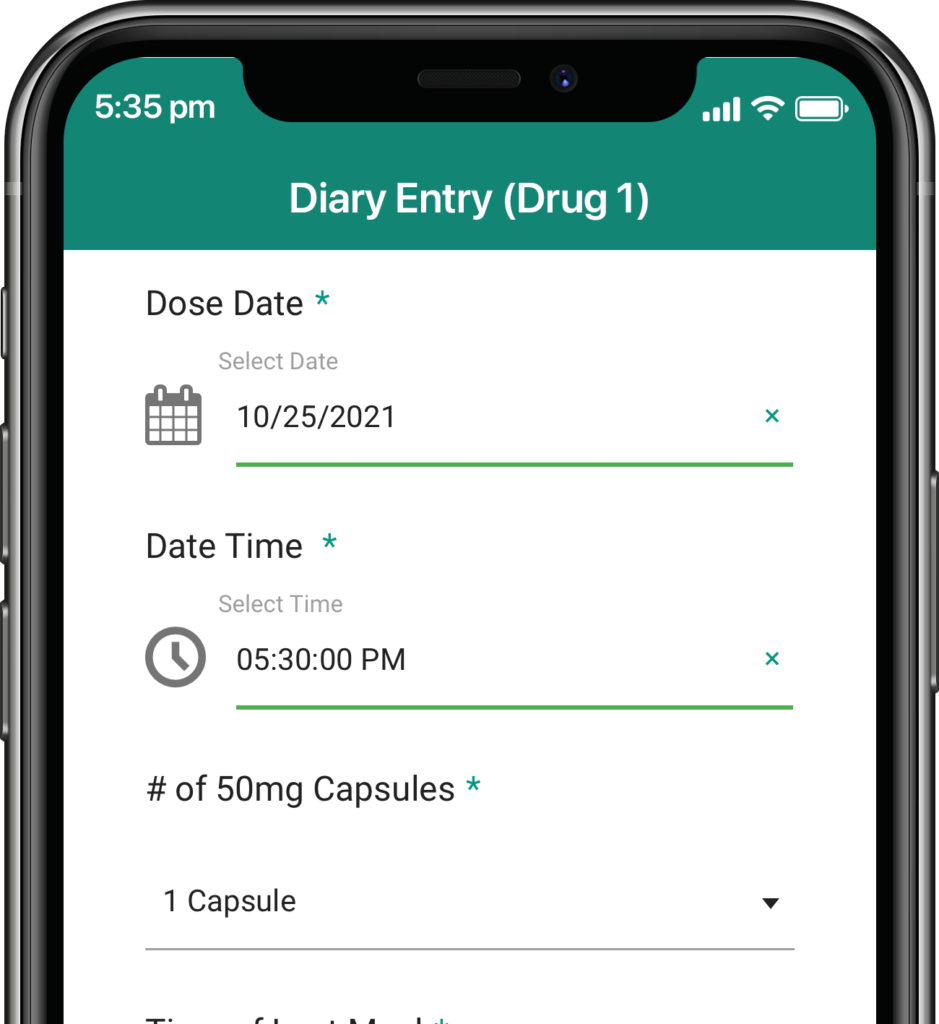
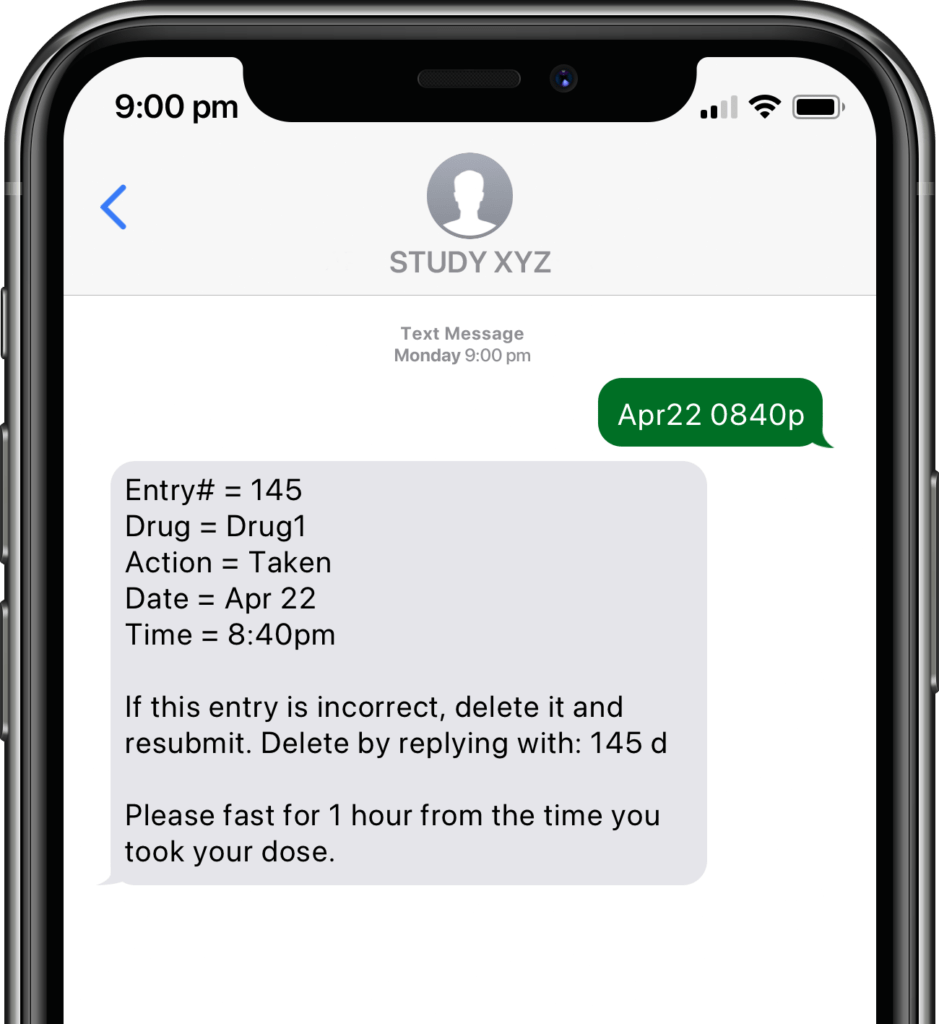
Participants submit or obtain information via text messaging available on ALL mobile phones. The user interface can also be on web or mobile app.
mComply Solution
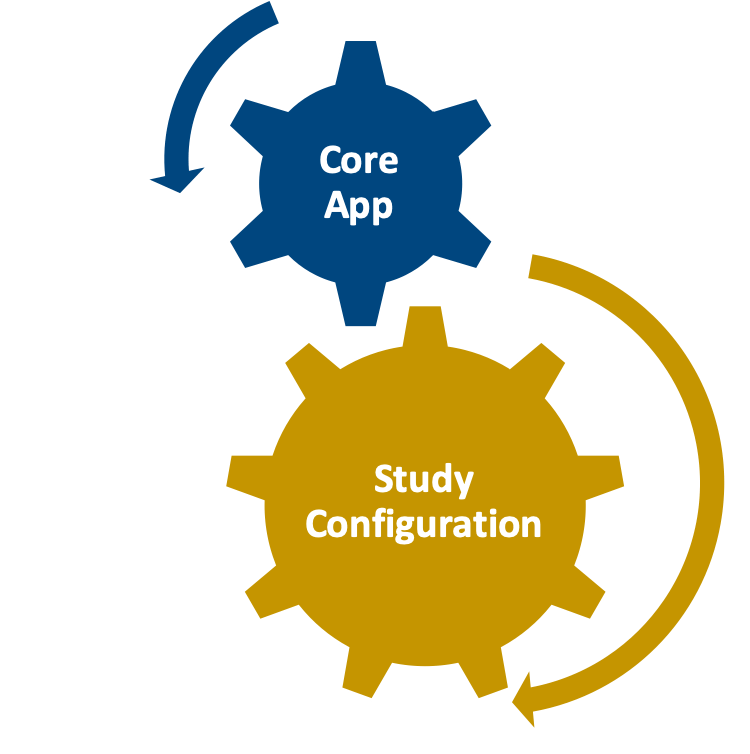
Validated, CFR Compliant
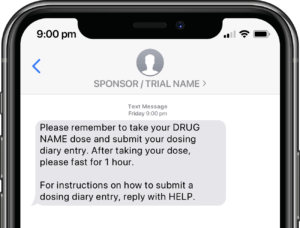
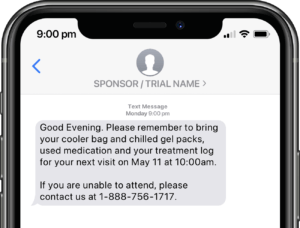
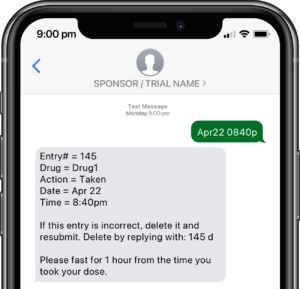
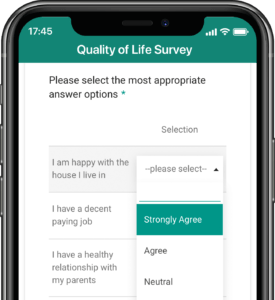
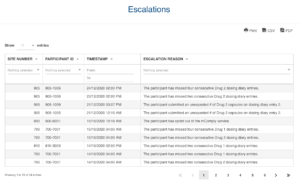
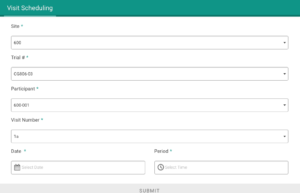
Application modules are customized to support your study protocol. These include interventions supporting study compliance and infrastructure processes such as recruitment, registration, dosing schedule, appointments, reminders, wellness checks and escalation workflows.
Participants submit or obtain information via text messaging available on ALL mobile phones. The user interface can also be on web or mobile app.
Health Organization

Web Portal
Based on user-access levels, team members can securely access all the data sent / received, export data and manage performance.