The Need for Creating Validated Custom Solutions
In the dynamic realm of clinical research, technology has proven to be a transformative force, optimizing efficiency, precision, and patient engagement. With stringent regulatory demands, the critical need for accurate data collection, and the inherent diversity of study protocols, the creation of validated custom solutions becomes an absolute necessity. This article delves into the critical steps involved in crafting validated solutions that align with regulatory standards such as CFR Part 11 and HIPAA for clinical research.
Understanding Validation and Regulatory Compliance
At its essence, validation guarantees the intended operation of software systems while adhering to predetermined specifications. In the domain of clinical research, validation plays a foundational role in maintaining data integrity and ensuring patient well-being.
Clinical research operates within strict regulatory frameworks to uphold data integrity, patient safety, and ethical principles. CFR Part 11 compliance, governed by the U.S. Food and Drug Administration (FDA), establishes guidelines for electronic records and electronic signatures. Adhering to CFR Part 11 standards assures precision, security, and reliability in electronic records.
Further enhancing these regulations, the Health Insurance Portability and Accountability Act (HIPAA) mandates the protection of patients’ protected health information (PHI) during its transmission and storage. Compliance with HIPAA guarantees the confidentiality and security of sensitive patient data.
Moreover, adhering to regional regulations is vital; Europe’s General Data Protection Regulation (GDPR) maintains information privacy and security. Additionally, the California Consumer Privacy Act (CCPA) empowers consumers with greater control over their collected personal information. It’s imperative to recognize that the landscape of regulations varies based on the geography of operations. salsa
In the realm of text messaging, the Telephone Consumer Protection Act (TCPA) and the Cellular Telecommunications Industry Association come into play, curbing unsolicited text messages and offering guidelines that further shield consumers. These collective regulations work harmoniously to fortify data protection and uphold ethical standards.
Creating Validated Solutions: A Methodical Approach
- Planning and Design: Initiating the process by identifying the precise requirements of the clinical research project is paramount. Collaboration with stakeholders such as researchers, healthcare professionals, IT experts, and study coordinators is essential to identify the necessary functionalities that support the study protocol. Emphasizing workflow optimization and patient engagement strategies contributes
- Validation Strategy: A well-defined validation plan serves as a strategic roadmap outlining the scope, objectives, and methodologies for ensuring compliance. This plan encompasses the validation of software functionalities, data integrity, security protocols, and user acceptance, guiding the entire validation process.
- System Configuration and Development: Establishing the system architecture and crafting the software solution according to the established requirements mark this phase. This may entail creating web-based platforms, mobile apps, and interfaces for medication reminder text messages. A key focus is on designing the system to ensure accurate data capture, real-time notifications, and the security of patients’ Protected Health Information (PHI).
- Validation Testing: Validation testing takes center stage, encompassing a series of executed tests scrutinizing the solution’s functionalities and performance. These encompass unit testing, integration testing, system testing, and user acceptance testing. Verification and validation activities certify that the solution operates as expected, ensuring seamless functionality and alignment with predefined criteria.
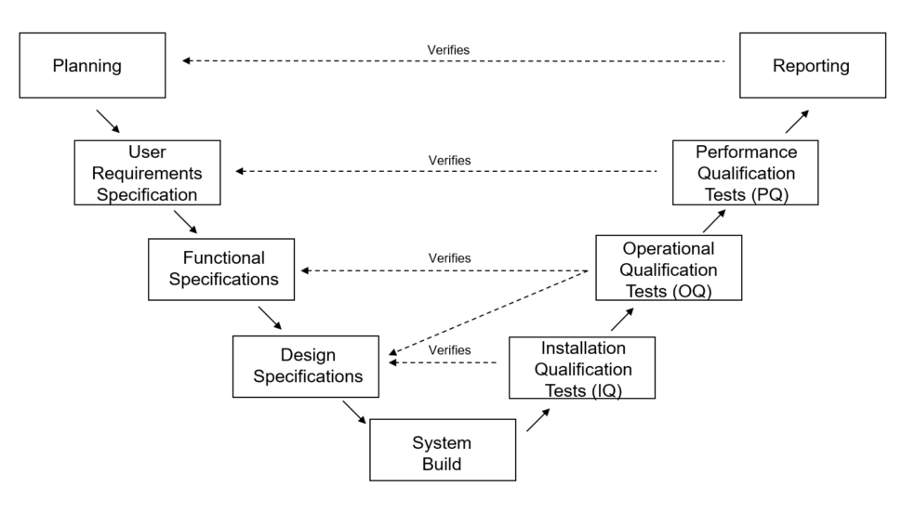
Documentation and Record-Keeping: Comprehensive documentation plays a vital role in the validation process, encompassing the formulation of test protocols, results, and the recording of any deviations encountered. This documentation serves as tangible evidence attesting to the solution’s adherence to CFR Part 11 compliance and other regulatory requisites.
- User Training: Empowering users, spanning researchers, healthcare professionals, site coordinators and administrators, through comprehensive training is integral. Ensuring their proficiency in navigating the validated solution, effectively utilizing its features, and upholding data integrity constitutes a cornerstone of this phase.
- Security and Data Protection: Establishing robust security measures is pivotal, safeguarding PHI in alignment with HIPAA regulations. Encryption, access controls, periodic audits, and secure data transmission protocols converge to fortify this initiative.
- Ongoing Maintenance and Monitoring: Continual monitoring of the solution’s performance, coupled with swift rectification of glitches and vulnerabilities, stands as a hallmark of this phase. Regular updates enhance functionality, and periodic reviews validate compliance with CFR Part 11 and HIPAA, reinforcing the solution’s credibility.
Crafting a validated custom solution for clinical research demands a comprehensive approach that harmonizes CFR Part 11 compliance, HIPAA security, and innovative technological features. By integrating elements like medication reminder text messages, web-based solutions, and mobile apps, researchers and healthcare professionals can elevate patient engagement, streamline data collection, and preserve data integrity. The process of developing such a solution necessitates meticulous planning, rigorous testing, and steadfast adherence to regulatory standards. As technology continually shapes the landscape of clinical research, validated solutions underscore the industry’s dedication to precision, efficiency, and patient well-being.
mobileHealthWorks, along with our parent company, Synegys, can implement validated custom solutions through its AI-powered health technology platform—mComply. These solutions artfully integrate text messaging technology, web-based applications, and mobile apps, providing an environment to support diverse study protocols.
Contact us to learn more about how we can help you with a validated custom solution to support your study protocol.