Improving Medication Adherence & Study Compliance
Compliance is an important determinant of clinical trials and can be improved through effective patient engagement. High adherence rates can be achieved by sending patients timely messages including appointment, medication and diary entry reminders and motivation messages. Moreover, diary entry submissions and other forms of data collection can be automated with the use of mobile phones.
With adherence rates in clinical trials ranging from 39% – 63% for prevention trials and 59% – 78% for treatment trials1, there is tremendous opportunity to improve compliance. A meta-analysis of trials of interventions to improve medication adherence showed increases in adherence in the range of 4% – 11%. Synegys estimates that a 1% improvement while maintaining statistical power would deliver nearly $400,000 in cost of capital savings for a typical Phase 2 trial having an average of 203 patients.2
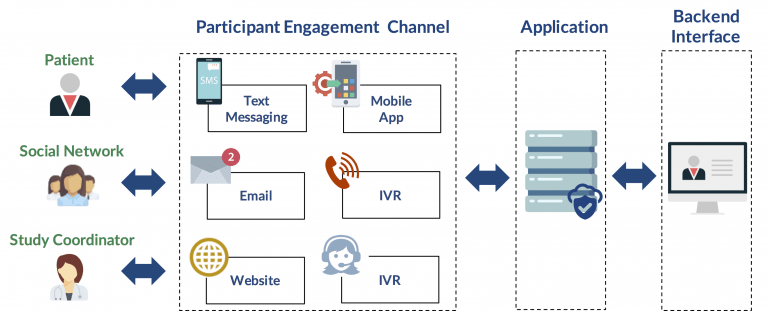
Given the pressures today to improve the cycle time of a drug release and reduce costs, companies require compliance tools such as Synegys’ mComply. mComply is a customizable, cloud-based system allowing sponsors to help patients follow a medication regimen and subsequently improve compliance. In fact, mComply offers a 97.5% medication adherence rate. It supports any front-facing, communications interface including any one or combination of text messaging (i.e. SMS), mobile app, email, interactive voice response, web portal or live agents.
With every study being unique, mComply is customized to support any study protocol requirement. Derived from over 15 years of experience, our patient engagement tools and processes help health organizations engage patients, improve outcomes and lower costs.
William Eng, Managing Director - Synegys
mComply is positioned as a complete solution to engage with patients after they have been enrolled in a trial. This includes handling patient registration (and opt-out) into the system, notifications and reminders, diary entries, and escalation workflow. Other compliance tools are available to improve patient recruitment and to collect data.
Key attributes of Synegys’ compliance tools are: 1) validated custom solution to support a specific study protocol; 2) 1-way or 2-way messaging support service coverage by over 1,200 mobile operators in 200 countries; 3) validated and compliant system that includes 21 CFR part 11, HIPAA, EU 2016/679 GDPR (General Data Protection Regulation) and local text messaging regulations and local privacy laws; and 4) comprehensive solution delivery to include training, patient consent forms, support for Ethics Committee documentation and the like.
For more information why medication adherence is important and how mComply can improve study compliance, ask for our brochure: mComply: Running Successful Clinical Trials – Adherence Delivered.
References
- Robiner, William N. Enhancing adherence in clinical trials. Contemporary Clinical Trials. 2005;26:59–77.
- Synegys. mComply: Running Successful Clinical Trials – Adherence Delivered. 2018.